CRISPR gene editing: revolution or evolution?
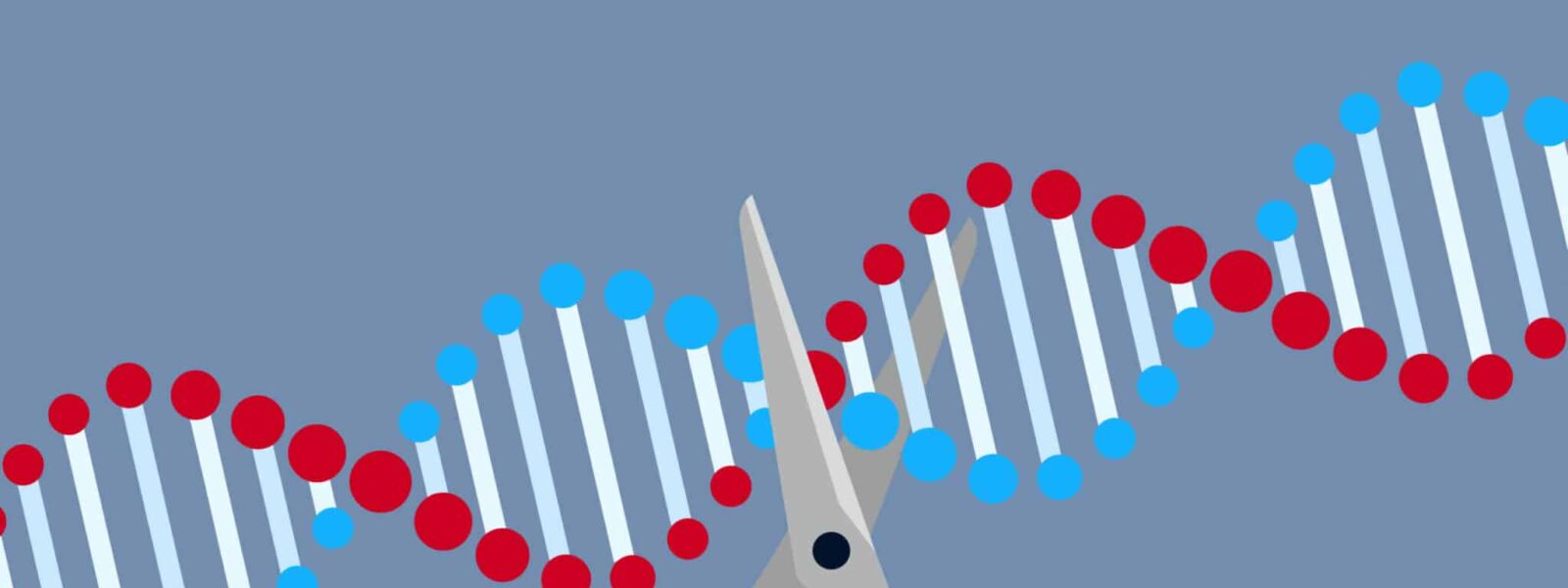
In 2020, the Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna for a technique they had invented only 8 years before. Known as CRISPR/Cas9 – or “molecular scissors” – they first presented the method to the world when they published their paper in prestigious journal, Science, in 2012. Since then, the technique has been touted as a revolution in the world of molecular biology and beyond.
INSERM researcher Dr. Erika Brunet at Institut Imagine, in the field cellular and molecular biology, uses the technique regularly in her laboratory. She says that whilst CRISPR/Cas9 has been cited as a revolution, previous techniques shouldn’t be forgotten. For her, it could not have happened had other researchers not paved the way.
As a scientist in the field of cancer research, how has CRISPR-Cas9 transformed biology?
Erika Brunet. CRISPR-Cas9 is a highly effective technique for cutting DNA at a precise location, allowing the user to replace the sequence cut out with another one; a fundamental basic of ‘gene editing’. Whilst this is not the first method of DNA alteration that exists, it is a highly effective, “easy to use” and flexible method. As such, it opens the door to many applications – most notably gene therapies, in which we could replace defective genes in patients to treat them for certain diseases.
There are also many research applications too, though. In my field, cancerology, we use CRISPR/Cas9 to seek new therapeutic targets. We cut out genes in cells to re-trace the steps of tumour growth. The tool its highly flexible, all that is needed is to order a desired RNA sequence or to make it – which is a surprisingly easy thing for a biologist to do!
For me, however, it is important to point out that CRISPR/Cas9 would not exist had it not been for the many years of research that had paved the way before it. Yes, the CRIPSR-Cas9 that won the Nobel Prize is the ‘new generation’ of molecular scissors. But it is a technique that is more of an evolution in molecular technology rather than a completely new idea. We don’t hear enough about that.
CRISPR is a fantastic technique as it works “in the blink of an eye” – the design of CRISPR/cas9 system for one DNA target only takes a few days. Also, before when we wanted to cut DNA in a precise location, we would be successful in 1 cell out of very ~1 million tested. With CRISPR, we are frequently successful in 1 cell per 100 – so the jump is enormous.
Previously, when we wanted to cut the DNA at a specific point, we succeeded in about one cell in a million.
In your research, you seek to better understand tumours. How do you use CRISPR-Cas9?
In my laboratory we study tumoral cells, specifically the process of how a normal cell becomes cancerous. Many cancers such as leukaemia and lymphomas develop because of an accidental genetic alteration in a process called ‘genomic translocation’. It happens when two chromosomes in a cell crossover and swap a long piece of their DNA with one another. Most of the time when this happens the cell will “cope” with this exchange of chromosome segment as it does not occur on an important gene sequence. But on some occasions a new ‘cancer’ gene called oncogene will be formed that will make the cells to “transform” and grow chaotically, eventually leading to a tumour.
We can use CRISPR/Cas9 to study how this process works, from the moment that a normal cell acquires the ‘genomic translocation’. To do so, we simply cut the DNA of a cell of origin of the disease (a blood cell for example), to recreate the new ‘cancer’ gene thus turning the healthy cell into a cancerous one. These cancer cells replicate uncontrollably, growing into a tumour, which we then put into a mouse for study. The important aspect is that, under experimental conditions, we can replicate the very same series of DNA ‘events’ that would happen in real-life and dissect the tumour process from the origin. This allows us to better understand how each DNA alteration of a cell can eventually become leukaemia, lymphoma, or any other cancer. Ultimately, we can identify new tumour markers and therapeutic targets.
What advantages does the CRISPR-Cas9 technique have in your field?
We work on specific cancers such as Ewing’s sarcoma, a bone cancer which mainly affects children and adolescents. Currently in most cases, the prognostic is very bad – the cancer metastasises (meaning it spreads to the rest of the body) in as many as 30% of patients. Whilst we have a hard time effectively treating Ewing’s sarcoma, we do know a fair amount about its origins. In ~90% of cases, the disease stems from a genomic translocation that happens when chromosomes 11 and 22 crossover accidentally. As such, we use CRISPR-Cas9 re-create the error by precisely cutting chromosomes 11 and 22 that can occur in cells at the origin of Ewing’s sarcoma tumours. Using combinations of different patient mutations induced by CRISPR/Cas9, we recently obtained a unique model of Ewing sarcomagenesis that should be valuable for the scientific community working on this particularly aggressive paediatric cancer.
What existed before CRISPR-Cas9?
At the very beginning, when I started my research in cell biology, I would use short pieces of DNA attached to chemicals to alter DNA. They were very niche and could be used to target multiple short DNA sequences. Next, there were what we called meganucleases and zinc-finger nucleases, which were a step up but still difficult to design and engineer – so not accessible to everyone. Importantly, however, using this ‘first generation’ of DNA nucleases we saw real achievements based on these techniques; zinc-finger nucleases are used to reach clinical trials for cures to HIV infection. Then, in 2010 we saw the arrival of TALENs. They were much easier to handle, being assembled in the laboratory in under three weeks with a simplified code to recognise each base pair of DNA. This really put nucleases on the map for widespread use. But two years later CRISPR/Cas9 arrived, knocking TALENs off their throne. Therefore, it could be said that others had done a lot of the leg work developing techniques and getting gene editing out there for numerous types of cells in different species, paving the way for CRISPR.